At-home COVID-19 testing kits have transformed the way individuals manage their health by allowing convenient, reliable access to testing. From Labcorp’s PCR test kits to iHealth’s COVID-19/Flu combined tests, options abound for varying needs and budgets. With free kits available and Emergency Use Authorizations in place, these tests aid in timely diagnosis during the pandemic. Delve into this comprehensive guide to make informed choices about at-home testing solutions.
COVID-19 Testing Options: Ensuring Accessibility at Home
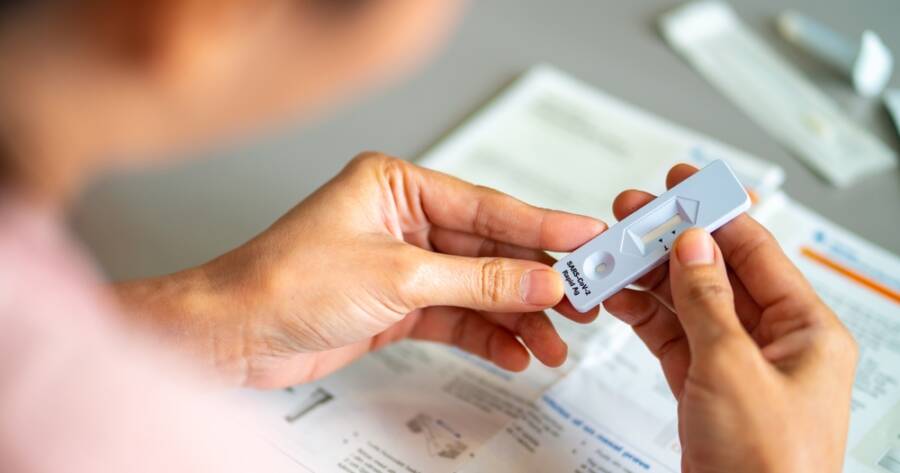
In recent times, the convenience of at-home COVID-19 testing kits has become a crucial aspect of managing health responsibly. Various options cater to different needs, with providers ensuring these kits are both reliable and accessible.
Labcorp, for example, offers an at-home COVID-19 PCR test collection kit priced at $79. This option allows individuals to collect a nasal swab at home and mail it back for laboratory analysis. Results are typically available online within 1 to 2 days ensuring timeliness in obtaining your results.
Options for Free and Low-Cost Test Kits
Beyond commercial avenues, U.S. residents have access to an allocation of four free at-home COVID-19 test kits per household. These tests offer rapid antigen results in less than 30 minutes without the need for lab drop-offs, providing great convenience and accessibility.
Reliable and convenient testing is key, especially as antigen tests are typically less reliable than PCR tests. It is recommended to perform multiple antigen tests to ensure accuracy.
Versatile At-Home Testing Solutions with iHealth
Further expanding the at-home testing landscape, the iHealth COVID-19/Flu A&B Rapid Test delivers a comprehensive approach by testing for COVID-19 and flu strains A & B simultaneously.
This 3-in-1 testing capability, which is seamlessly executed with one sample, makes differentiating between these viral infections significantly easier. The process is straightforward, requiring only simple steps to perform, and results are produced in as short a time as 15 minutes.
Regulatory Approvals and Considerations
Although not fully FDA approved, both Labcorp and iHealth at-home kits have obtained the Emergency Use Authorization (EUA), allowing these tests to be a part of public health responses during the pandemic.
The EUA status highlights their trusted role in enabling at-home diagnostics across various age groups. Despite this, individuals should consult healthcare providers for additional guidance and recommendations after obtaining any test results.
The Importance of Timely COVID-19 Testing
Amidst an ongoing health crisis, timely diagnosis and understanding of COVID-19 status uphold a societal duty to curb the spread of the virus. Whether through free governmental services, purchased PCR tests, or innovative at-home kits like those from iHealth, individuals are encouraged to actively utilize these tools.
It’s recommended to follow guidelines such as those from the CDC and seek medical advice if necessary. Staying informed and proactive about available testing options can make a significant difference in managing public health and personal safety. Whether preparing for travel, attending gatherings, or simply feeling under the weather, accessible COVID-19 tests present an invaluable utility in today’s world.
Why You Should Learn More About At-Home COVID-19 Testing Today
With the evolving nature of the COVID-19 pandemic, the capabilities and types of available tests are continually adapting. Understanding the nuances between antigen, PCR, and combination tests like those covering flu strains can better prepare individuals for varied scenarios, ensuring informed choices are made concerning health and safety. Additionally, insights into regulatory measures and test efficacy during the public health emergency can shed light on the best practices for medical decisions.
Sources
Timely Labcorp Testing Insight